Single-use simplified
Single-use process components and assemblies with biopharma, life science and cell therapies in mind.
Single-Use Consumable Solutions

SUT Assemblies & Custom Tube Kits
Critical fluid management supporting bioreactor, cell culture, and cell therapy applications.
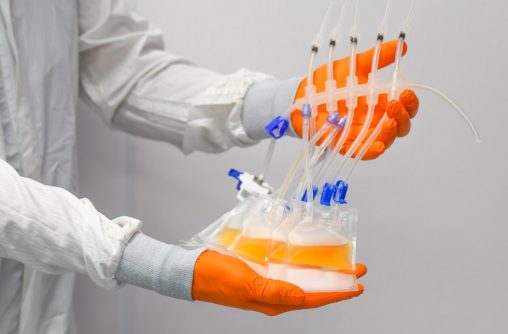
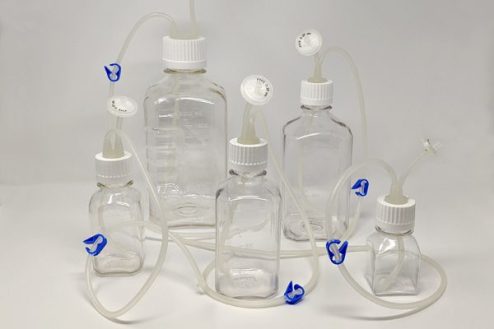
Bottle & Tube Assemblies
One Piece Centrifuge Tube Assembly with customizable options, offered in 15ml, 50ml, 250ml, and 500ml. Complete bottle assembly for efficient aseptic transfer with 100% fluid path integrity.
Molded Sanitary Connectors & Gaskets
Molded platinum-cured silicone and TPE sanitary connections for critical fluid transfer applications.


Cell
Therapy
The emergence of cell and gene therapies has created unique fluid-handling challenges for traditional SUT suppliers. WHK specializes in manufacturing complex tube kits that address these particular applications.

Overmolding
Overmolded medical device components are often used in combination with our W-TPE™ Tubing to create many types of Single-Use Systems for the biopharmaceutical and life sciences industries.


Intelligent Solutions Need Customizable Services
Injection Molding
Continually investing in injection molding capabilities ensures that we evolve with your needs.
Design Services
Every aspect, from initial device design to full-scale production, can make or break a product’s success.
Device Assembly
Sourcing and production of custom tube kits and device assemblies help increase productivity, lessen costs.
Tubing Extrusion
Planning and preparing for your needs are what make us the supplier of choice for tubing extrusion.

Disclaimer: User is responsible for determining suitability and safety of all products for intended use. Information as supplied on this site is intended to provide guidance only. WHK BioSystems disclaims all liability regarding product fitness for use. WHK BioSystems has also relied on raw material suppliers for a portion of the information and compliance statements contained on this site.
WHK BioSystems, LLC, maintains an ISO 13485:2016 registered quality management system. It operates Class 7 cleanroom manufacturing facilities.
CLASS 7
Cleanroom Manufacturing
ISO 13485
Quality Management System
Made In the USA
American Manufacturing


